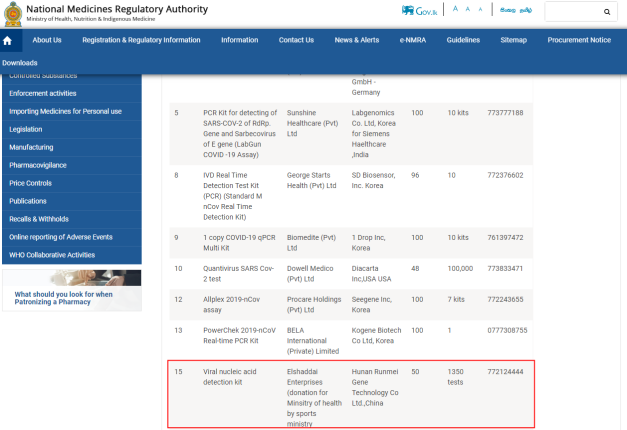
In early 2020, the Viral nucleic acid detection kit independently developed and produced by Hunan Runmei Gene Technology Co., Ltd. was certified by the Sri Lanka National Medicines Regulatory Authority.
The National Medicines Regulatory Authority (NMRA), plays a leading role in protecting and improving public health by ensuring medicinal products available in the country meet applicable standards of safety, quality and efficacy. The Authority regulates medicines, medical devices, borderline products, clinical trials and cosmetics. The National Medicines Quality Assurance Laboratory (NMQAL), charged with ensuring quality of medicinal products, also functions under the purview of the NMRA.
As a company specializing in the production of medical devices, in order to standardize the company's product quality management, so as to gain a competitive advantage for the company and achieve the company's mission, under the guidance of the expert group, the company consciously abides by the requirements of the detailed rules in accordance with the requirements of the production licensing rules. Implement the requirements of the drug production license management method within the company, scientifically manage the work of various departments, analyze the status of the enterprise, accept the review and certification of the review teacher, and finally pass the Sri Lanka National Medicines Regulatory Authority Certification.
The company's quality management system certification in Sri Lanka passed this time, which fully demonstrates that our products have a very good quality assurance and are liked by customers abroad. On the other hand, it also fully demonstrates the spirit of unity and courage of employees of the company. It is the result of the joint efforts of all employees of the company. I believe we will achieve even more impressive results in the future!


