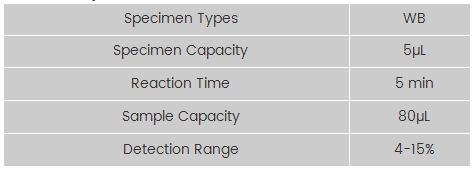
Technical Specifications
Product Advantage
The sample requirement is low, only 10μL of serum/plasma is required
Clinical significance
One of the most sensitive indicators to detect early AMI, exclude early AMI negative
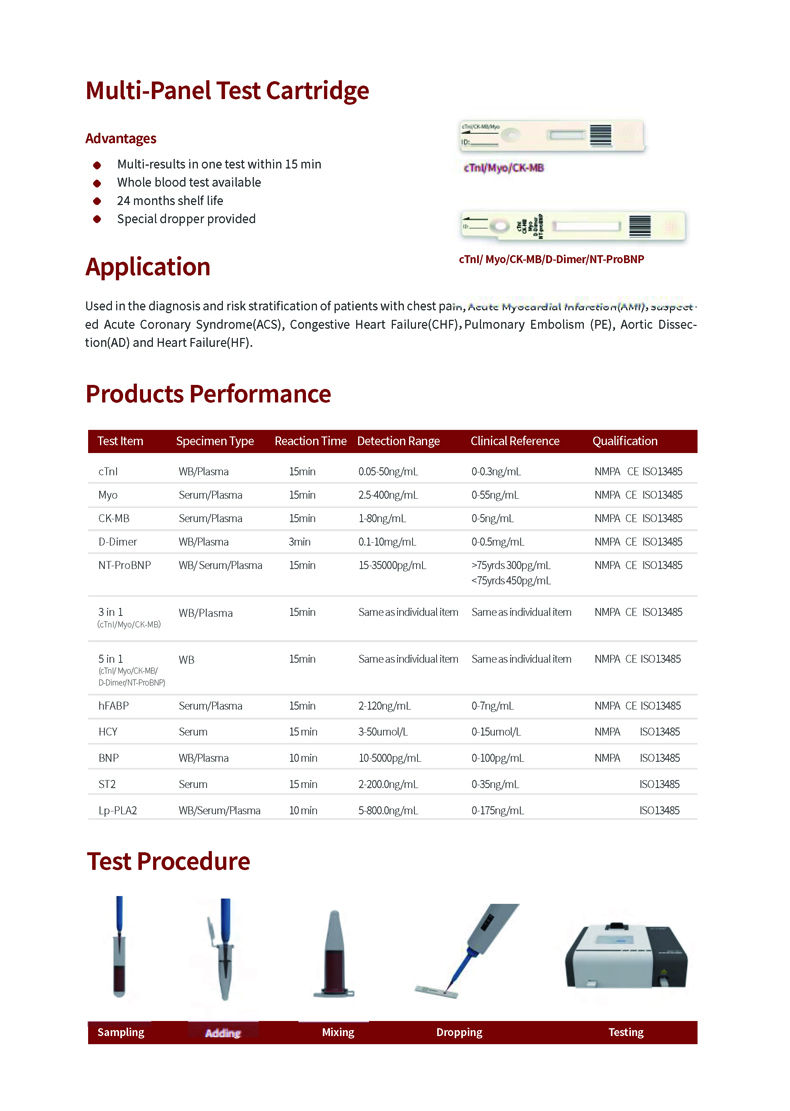
Application
ICU, Respiratory Medicine, Emergency Department, Cardiology, Laboratory, Emergency Car
Reference
1. Le Tao. The clinical application and evaluation of the triad rapid diagnosis to myocardial infarction. Journal of Chinese Clinical Medicine. 2005. 140: 15239- 15240
2. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction[J]. J Am Coll Cardiol. 2000. 36(3):959-969.
3. Levinson SS. The nature of heterophilic antibodies and their role in immunoassay interference. [J]. Clinical Immunoassay. 1992. 15:108-15.
Product Description
-
Product Introduction
This product is used to detect concentration of Myoglobin (Myo) in human serum and plasma. Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates. In human body, myoglobin is only found in the bloodstream after muscle injury. While muscle injury occurs, myoglobin could be released into blood rapidly and exceeds the normal level in about 1 hour. Thus myoglobin is a sensitive marker for muscle injury, making it a potential marker for heart attack in patients with chest pain. However, elevated myoglobin has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cardiac Troponin, ECG, and clinical signs should be taken into account to make the diagnosis[1- 3].
FAQ
-
1.We are based in Guangdong, China, start from 2021,sell to Africa(30.00%),South Asia(15.00%),Mid East(10.00%),Domestic Market(10.00%),North America(5.00%),South America(5.00%),Eastern Europe(5.00%),Southeast Asia(5.00%),Oceania(5.00%),Eastern Asia(5.00%),Western Europe(5.00%),Central America(5.00%),Northern Europe(5.00%),Southern Europe(5.00%). There are total about 51-100 staffs in our office.
2. How can we guarantee quality?
Always a pre-production sample before mass production;
Always final Inspection before shipment;
3.What can you buy from us?
Test kit,In Vitro Diganositc,Disposable Samplers,Rapid test kits,Swab
4. Why should you buy from us not from other suppliers?
We focus on producing virus nucleic acid detection kits, Disposable samplers, IGG/IGM rapid test kit, PCR test kit etc.The products approved CE, FDA, SGS, ISO13485.More than 200 staffs, 10 production line.
5. What services can we provide?
Accepted Delivery Terms: FOB,CFR,CIF,EXW;
Accepted Payment Currency:USD,EUR,HKD,CNY;
Accepted Payment Type: T/T,Credit Card,PayPal, Western Union,Escrow;
Language Spoken:English,Chinese,Spanish,Japanese,Portuguese,German,French,Russian,Korean
6.Can I have samples of this goods to test the quality?
Yes, free sample is available.
7.What's the delivery time and can you lower the price?
It depends on your order. The delivery time is within 3-4 days. If you have large quantities,the price and delivery time can be negotiable.
8.Can I customize the packaging and the logo?
Yes, we could offer customized logo for you.
9.What's the shipping method?
We often use DHL, FedEx, UPS, TK,EK,LH to ship our products.
10. The PCR test kit is required to be stored at a temperature between -25 degrees Celsius and -15 degrees Celsius. How did you do it?
Most of our PCR detection reagents are transported in an incubator, and the temperature can be maintained at -20 degrees Celsius for about 12 days, which can fully meet the general air transportation time limit.







