During special time when the global epidemic continues to spread, in order to more effectively support the international community's jointly response to the global public health crisis,we are hereby further strengthening the quality supervision and normalize export orders of epidemic prevention materials. On April 25, the Ministry of Commerce、the General Administration of Customs and the State Administration for Market Regulation released a bulletin No. 12 of 2020 on Further Strengthening the Supervision over the Export Quality of Epidemic Prevention Materials.
The relevant measures are announced as follows:
The statement indicates that, since April 26th, export enterprises of new coronavirus detection reagents,whose products have obtained foreign standard certification or registration must submit electronic or written declarations (declaration of exportation for medical products) for customs declaration, promising that products meet the quality standard and safety requirements of the importing country (region).Then customs will check and release against the list of manufactures with foreign standard certification or registration provided by Commerce Department (dynamically update from www.cccmhpie.org.cn website of China Chamber of Commerce for Import and Export of Medicines and Health Products)
On June 3, China Chamber of Commerce for Import and Export of Medicines and Health Products updated list of medical products manufacturers with foreign standard certification or registration.Hunan Runmei Gene Technology Co., Ltd. Is among them.That is to say,our new Coronavirus products have been authorized exportation.
The products of the series has been approved by the European Union and Department of Commerce, this will enable us to contribute to the prevention and control of COVID-19 globally.
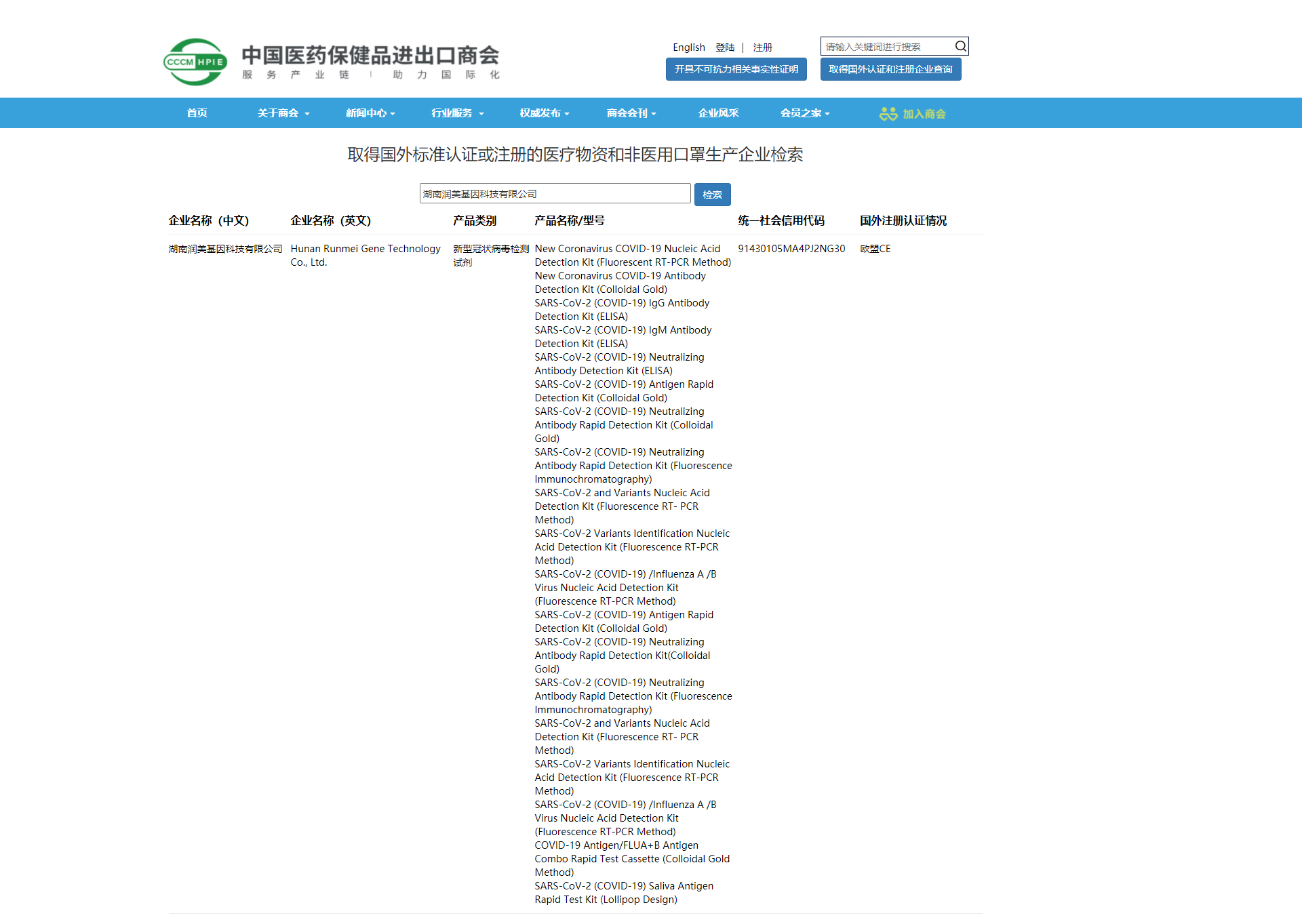
New Coronavirus COVID-19 Nucleic Acid Detection Kit(Fluorescent RT-PCR Method)、New Coronavirus COVID-19 Antibody Detection Kit (Colloidal Gold)、Virus Nucleic Acid Extraction Kit or Nucleic Acid Detection Kit、Single-use samplers and so on.
The products can be used in wide ranges and various locations.and Rapid and high-throughput screening of COVID-19 patients and suspected cases can be achieved in hospitals, communities, clinics, schools, moving monitoring stations, stations, customs and CDC



