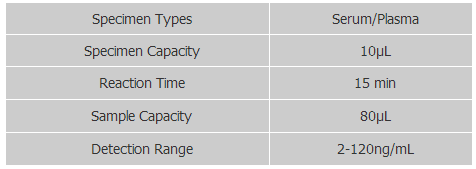
Technical Specifications
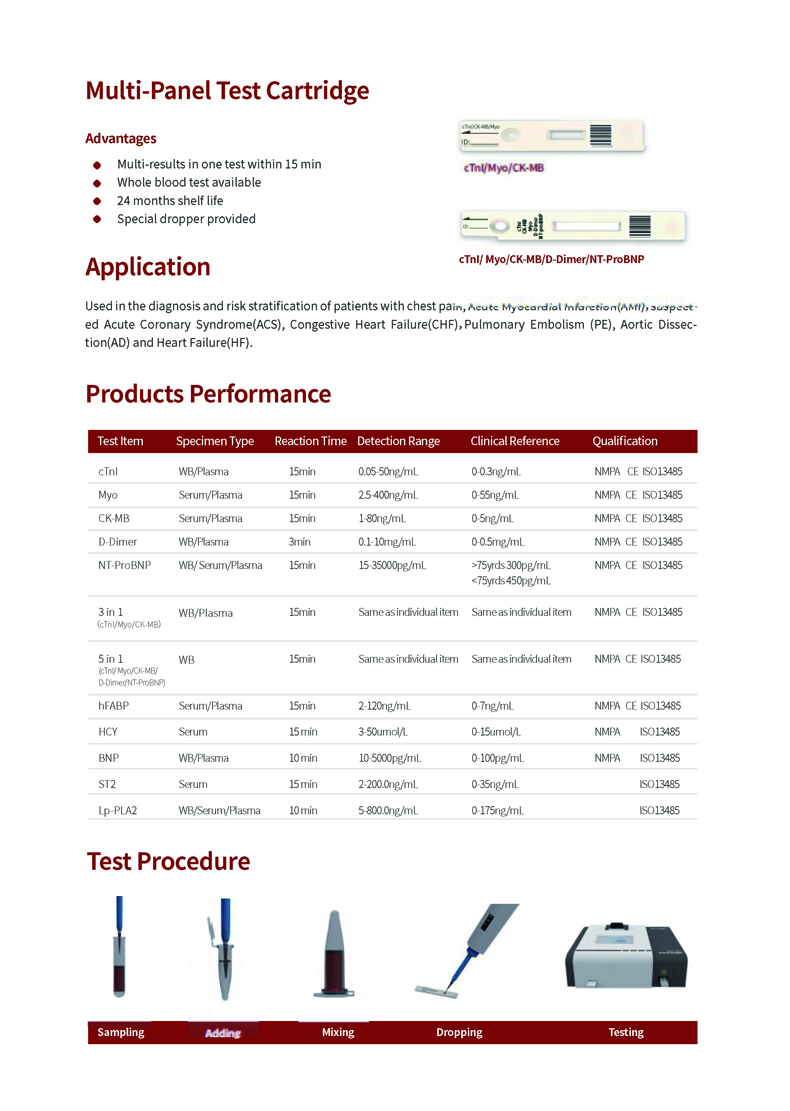
Product Advantage
Advantage
The sample requirement is low, only 10μL of serum/plasma is required
Clinical significance
√ Detect early AMI with high sensitivity
√ Assess the area of myocardial infarction, detect myocardial infarction reperfusion and recurrence, etc.
√ As an important indicator of brain injury, nervous system disease, carbon monoxide poisoning diagnosis, and treatment effect evaluation
Application
ICU, Respiratory Medicine, Emergency Department, Cardiology, Endocrinology, Nephrology
Product Description
-
Product Introduction
hFABP is a kind of new cytoplasmic cell in cardiac that has high specificity, mainly expressed in cardiac. Once myocardial ischemic injury emerging, hFABP can be detected from the first to the third hour before pectoralgia happens, reach the top level after the sixth to the eighth hour, and recover to normal level from the 24th hour to the 30th hour.
hFABP is a kind of soluble protein with relatively low molecular weight, it is widely found in human and animal multiple histocyte. At present, there are at least nine types of such proteins, such as small intestine protein, liver protein, kidney protein, heart protein, and so on. hFABP is one of these proteins. It is specific to the myocardial tissue, about 4% to 8% of the heart soluble protein. The expression level is extremely low or not expressed in other organizations. When myocardial ischemia is hypoxic or impaired, hFABP can be rapidly released into the blood. Therefore, hFABP is expected to develop an important index for early diagnosis of AMI and judgment of efficacy and prognosis of AMI thrombolysis.

FAQ
-
1.We are based in Guangdong, China, start from 2021,sell to Africa(30.00%),South Asia(15.00%),Mid East(10.00%),Domestic Market(10.00%),North America(5.00%),South America(5.00%),Eastern Europe(5.00%),Southeast Asia(5.00%),Oceania(5.00%),Eastern Asia(5.00%),Western Europe(5.00%),Central America(5.00%),Northern Europe(5.00%),Southern Europe(5.00%). There are total about 51-100 staffs in our office.
2. How can we guarantee quality?
Always a pre-production sample before mass production;
Always final Inspection before shipment;
3.What can you buy from us?
Test kit,In Vitro Diganositc,Disposable Samplers,Rapid test kits,Swab
4. Why should you buy from us not from other suppliers?
We focus on producing virus nucleic acid detection kits, Disposable samplers, IGG/IGM rapid test kit, PCR test kit etc.The products approved CE, FDA, SGS, ISO13485.More than 200 staffs, 10 production line.
5. What services can we provide?
Accepted Delivery Terms: FOB,CFR,CIF,EXW;
Accepted Payment Currency:USD,EUR,HKD,CNY;
Accepted Payment Type: T/T,Credit Card,PayPal, Western Union,Escrow;
Language Spoken:English,Chinese,Spanish,Japanese,Portuguese,German,French,Russian,Korean
6.Can I have samples of this goods to test the quality?
Yes, free sample is available.
7.What's the delivery time and can you lower the price?
It depends on your order. The delivery time is within 3-4 days. If you have large quantities,the price and delivery time can be negotiable.
8.Can I customize the packaging and the logo?
Yes, we could offer customized logo for you.
9.What's the shipping method?
We often use DHL, FedEx, UPS, TK,EK,LH to ship our products.
10. The PCR test kit is required to be stored at a temperature between -25 degrees Celsius and -15 degrees Celsius. How did you do it?
Most of our PCR detection reagents are transported in an incubator, and the temperature can be maintained at -20 degrees Celsius for about 12 days, which can fully meet the general air transportation time limit.







